Shortages of medical devices can occur for many reasons, including manufacturing and quality problems, geopolitical issues, natural disasters, delays, public health emergencies (PHEs), and discontinuations. The Office of Supply Chain Resilience (OSCR), in the CDRH Office of Strategic Partnerships & Technology Innovation (OST), is responsible for managing the FDA’s activities […]
Papers
Medical Device Reprocessing Market by Type (Reprocessed Medical Devices), Device Category (Critical- Devices, Semi-Critical Devices, Non-Critical Devices), Application (Cardiology, Gynecology, Gastroenterology, Anesthesia) – Global Forecast to 2027 Read more…
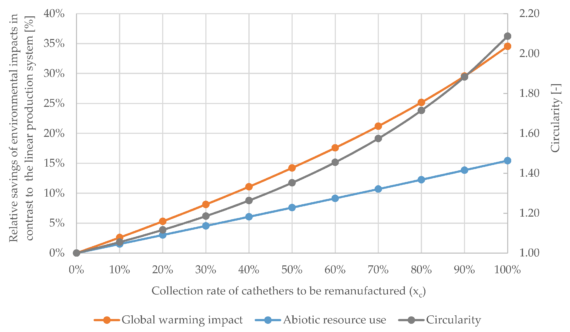
A 2021 study conducted by the Fraunhofer Institute for Environmental, Safety, and Energy Technology (Fraunhofer) analyzed the environmental impacts of the medical remanufacturing of Electrophysiology Catheters. By using reprocessed catheters as an alternative to newly manufactured ones, the study revealed they reduced global warming impact by 50% and ozone depletion […]
[McKinsey & Company] Circularity refers to practices that optimize resource use and minimize waste across the entire production and consumption cycle, emphasizing sustainability and economic efficiency. Read more…
A circular economy involves maintaining manufactured products in circulation, distributing resource and environmental costs over time and with repeated use. In a linear supply chain, manufactured products are used once and discarded. In high-income nations, health care systems increasingly rely on linear supply chains composed of single-use disposable medical devices. […]